Work plan
Overall structure of the workplan
NOVA's ultimate goal is to develop marketable bioactive coating solutions. Therefore, the work plan is structured to efficiently achieve this goal. NOVA is divided into 8 work packages (WP1-8). The synthesis, optimisation and production of antimicrobial coatings and nanoscale additives is the main objective of WP2. WP3 will define use cases, e.g. a variety of surfaces (glass, metal, alloys, ceramics, textiles, plastics) and everyday conditions under which the selected coatings will be tested. WP2 and WP3 will jointly adapt the most promising antimicrobial coatings, while WP5 will evaluate their effectiveness. The most promising coatings will in turn be tested for human safety (WP4) and sustainability (WP6). WP7 will guide this development and provide data science support and predictive modelling to consortium members to accelerate results. WP1 oversees project management in NOVA and covers administrative and scientific coordination. WP8 deals with dissemination and communication as well as exploitation of the results.
.png)
NOVA workflow and positioning within the TRL scale
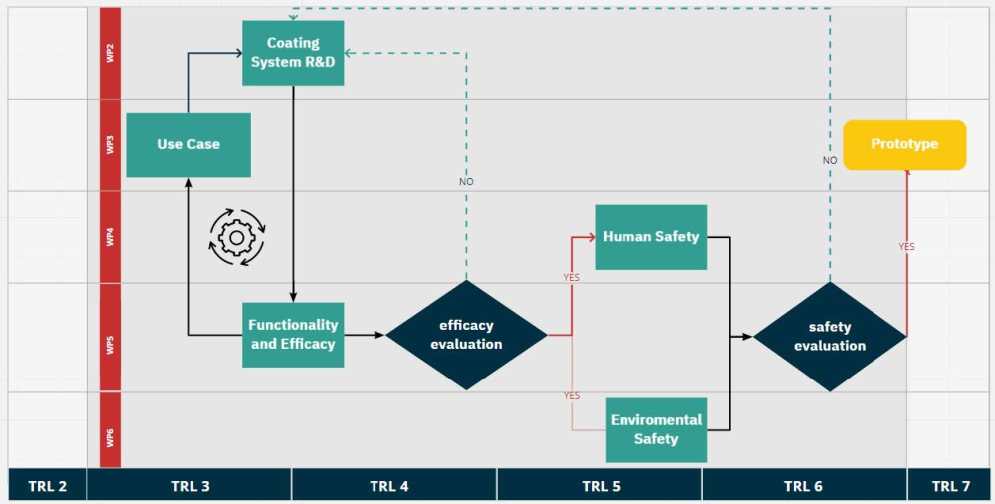
The Work Packages in a nutshell

WP1 will ensure the administrative and financial coordination of the project, incorporation of project management practices, effective means for quality control of the project, timely and effective execution of the work-plan and achievement of deliverables, effective communications with the EU Commission and partners and organization of consortium meetings, as well as facilitating the collection, tracking and dissemination of knowledge.
WP1 includes the following objectives:
- to execute the administrative and financial coordination of the project
- to define measures and procedures for Project Quality Management
- to facilitate close interaction and communication between the project partners
- to coordinate legal and IPR issues for the whole NOVA value chain
Lead Beneficiary: DECHEMA
On the one hand, WP 2 will develop biopolymer-based sprayable coatings that can adapt to any type of surface and have inherent antimicrobial properties and the ability to be loaded with other developed antimicrobial agents. On the other hand, more durable light-activated coating solutions in the form of additives and additional potentiating factors will be developed.
Intensive physicochemical characterization of the coatings and coating additives will be carried out to provide the necessary information for in-silico modelling to predict future coating behaviour in WP7.
WP2 includes the following objectives:
- to provide combinations of these antimicrobial coatings that can meet a wide range of potential needs for specific use cases.
- to optimize all coating formulations with respect to the input from WP4-6
- to establish a sustainable synthesis of the coating components for the lead candidates
- to provide the test materials for WP4-WP6 and the data for WP7 and, at the same time, create a feedback loop with WP3 for the potential applications and device applications (sprays, coating robots)
Lead Beneficiary: Spartha Medical

In WP3, the industry partners will define and elaborate the use cases and specifications that will be incorporated in WP2 for optimised coating developments. A feedback loop will be formed between WP2, WP3, and WP5 for iterations of the coatings, which will lead to the design and development of specific coating application tools in WP3. Overall, the aim is to develop a safe range of coatings for use on textiles, in public spaces, in deep-cleaned medical spaces and on glass or plastic used touchscreens.
WP3 includes the following objectives:
- to perform technology screening within NOVA and define use case criteria and needs
- to develop prototypes for application of coatings
- to validate coatings and application methods developed for commercialization.
Lead Beneficiary: AkzoNobel
WP4 deals with biological in vitro investigations on nanocoated surfaces developed in WP2 and WP3. The main objective is to accompany and to facilitate the decision for suitable, i.e., safe candidates for further use. First, robust biocompatibility tests and secondly organ-specific tests (advanced skin and immune modules) will be conducted. Suitable coatings for a targeted use revealed during the NOVA project will be further investigated in appropriate animal experiments (INSERM).
WP4 includes the following objectives:
- to evaluate biocompatibility (cytotoxicity) of coatings developed in WP2 / WP3 (iterative assessment)
- to establish new, reliable cell-based test strategies for immunotoxicity to conduct organ specific safety evaluation using I) advanced skin models and II) in vivo testing following the 3R principle
- to provide the test materials for WP4-WP6 and the data for WP7 and, at the same time, create a feedback loop with WP3 for the potential applications and device applications (sprays, coating robots)
- to identify gaps in the OECD guidelines and elaborate missing tests
Lead: Fraunhofer IKTS


WP5 aims to develop and use testing strategies that support coating invention and development activities and allows a predictive estimation of the antimicrobial action to be expected when they are deployed in practice.
To achieve objectives 1 and 2, the activities of participants 2 (Fraunhofer) and 12 (IMSL) will focus initially on supporting new coating invention and development by providing a harmonised testing programme to the participants of WP2. The cascade of testing and the model species to be employed will be selected during months 1 – 3 (bacteria, fungi and bacteriophages). The purpose of the screen will be to select coatings / coating technologies that can be progressed for further development.
Candidate systems will then be tested using conditions that reflect intended areas of use such that
coating / application / material matching can be achieved.
WP5 includes the following objectives:
- To provide a service that supports the invention and development of the novel antimicrobial surfaces by screening candidate systems for activity against a representative range of target species in a rapid manner that allows for robust
comparisons of relative efficacy to be made. - To assess those candidates that have been selected for progress for activity under conditions that simulate better their intended use. Both assessment systems are intended to form part of a ‘feed-back loop’ to both inform on system design and help to optimise it and allow the development of claims that are realistic rather than aspirational.
- Extend the development of the models used for Objective 2 (and to a lesser extent, Objective 1) above to improve their compliance with the real-world conditions they are intended to reflect.
- Extend the simulations to encompass mammalian viruses as, although the models will be functionalised for bacteriophage surrogates of them, there are a number of technical (as well as logistical and economic) hurdles that, to date, have prevented this being done for mammalian species.
- To develop and use testing strategies that support coating invention and development activities and allows a predictive estimation of the antimicrobial action to be expected when they are deployed in practice.
Lead: IMSL
The main objective of WP6 is to ensure the sustainable innovation of the antimicrobial coatings developed in WP2 and WP3. The whole life cycle from a sustainable synthesis until the end of life of the coatings will be considered. To ensure all aspects are considered during all stages of the development, a “Biocide-Safe and Sustainable by Design (SSbD)” strategy will be developed for the antimicrobial coatings, building on the Chemicals Strategy for Sustainability Towards a Toxic-Free Environment” by the EC, the EU Mapping study for the development of Sustainable-by-Design criteria, and the safe-by-design (SbD) concepts developed and applied in EU projects such as NanoReg2.
Additionally, this strategy will specify and apply the general SSbD concept and criteria developed by the EC (which will be communicated in late 2022) to antimicrobial coatings.
WP6 includes the following objectives:
- to develop the “Biocides-SSbD strategy and apply it to the NOVA-coatings
- to assess the sustainability of the NOVA-coatings
- to assess the environmental stability and release of the coatings
- to perform a toxicity screening of the antimicrobial coatings
Lead: Empa

WP7 objective is to provide to other members of the consortium, in particular the ones involved in WP2 and WP3, a data science support and in-silico algorithmic/predictive models to fast-track their research work. The data science support will help the contributors of WP2 and WP3 analyse and put in perspective the results of the experiments and research they conduct, and in particular the contribution of each internal/external factor and parameter of the coatings and coating’s production processes they test.
The predictive models will predict some key expected properties of the coating materials, notably their antimicrobial effect, their stability, some of their mechanical properties and their biocompatibility, from a combination of intrinsic components’ properties and production parameters. A specific focus will be made, for the second half of the project, on the impact of the nanoparticles on these key properties.
WP7 includes the following objectives:
- to fast-track research through in-silico modeling by simulating outcomes
- to empower the consortium to make better decisions with quantifiable, data-driven evidence
Lead: Preste
In WP8 targeted communication activities will be utilized to maximise the dissemination and exploitation of the project results. The main goal of WP8 is to implement and sustain the project activities during and after the funding phase of NOVA.
WP8 includes the following objectives:
- Targeted communication to and involvement of all relevant stakeholders through implementation of a Communication and Dissemination Strategy (CDS)
- The development of business plans for each use case in WP3 to implement and sustain the coatings development after the funding phase up to market maturity. Therefore, the initial concept for a business plan, described within this proposal, will be further elaborated including legal protection and trademark protection
- Achieving user acceptance of the newly developed (nano)coatings. Preparation of a regulatory roadmap and initial steps taken to obtain regulatory clearance. Targeted measures for communication and exchange with relevant stakeholder groups will be implemented
- Storage, exploitation and long-term preservation of data generated within NOVA according to the FAIR principles trough generation and implementation of a DMP
Lead: DECHEMA

